Cardiovascular disease is a leading cause of death in the United States, and heart failure is a substantial contributor to this statistic. Innovative solutions are desperately needed to improve the quality of life and outcomes of advanced heart failure patients who have limited treatment options.
Development of new therapies to treat health issues like heart failure depends on rigorous clinical trials to demonstrate that they are safe and effective. The current practice of finding potential trial candidates requires substantial manual effort from trial coordinators to sift through hundreds of patient charts, making identifying appropriate candidates like searching for a needle in a haystack. This outdated process delays clinical trial enrollment and can delay patients’ access to lifesaving therapies.
At egnite, we identify potential clinical trial candidates using AI and big data processing to screen hundreds of thousands of patients against trial parameters. Our Trial Accelerator solution provides hospitals and researchers with curated lists of patients meeting specific clinical trial criteria.
Thus far, our solution has improved workflow efficiencies by 55% and demonstrated a 50% increase in enrollment at a participating site.
Most recently, we announced an exciting strategic partnership with Ancora Heart, a medical device company specializing in transcatheter therapies for heart failure. Ancora has sponsored a new module at select sites within egnite’s CardioCare platform to help providers accelerate clinical trial enrollment for its CORCINCH-HF clinical trial for patients with systolic heart failure.
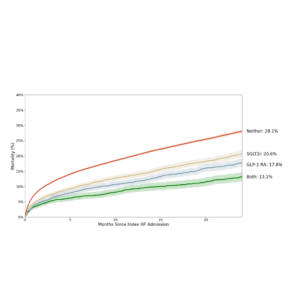
egnite also recently announced new heart failure research using egnite’s de-identified database of over 5.2 million patients with cardiovascular disease at the Heart Rhythm Society Annual Meeting. These data, from 20 leading heart programs, uncovered wide variability in care along with marked survival benefits associated with guideline-directed therapy.
There is great potential for new digital technologies to transform how clinical trials are designed and managed, and we look forward to improving clinical workflow and patient access to novel therapies. As egnite continues to innovate new technologies, we remain committed to improving patient outcomes and taking on the big challenges in healthcare by leveraging big data and AI.